
Dr. Anthony Haynes
Department of Chemistry, University of Sheffield


|
Dr. Anthony HaynesDepartment of Chemistry, University of Sheffield |

|
|
|
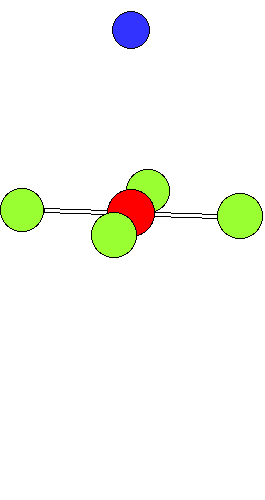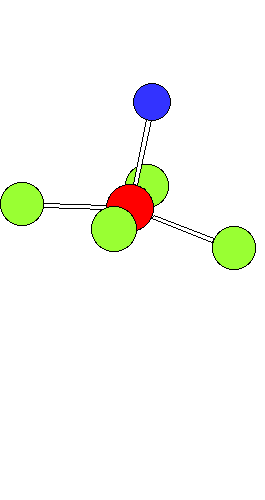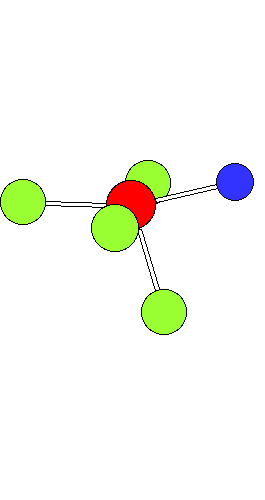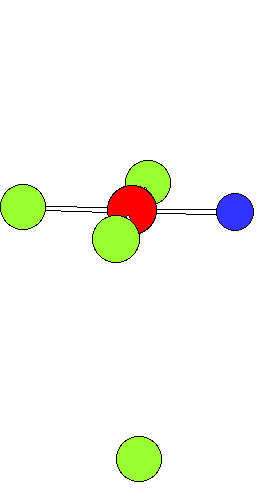
Ligand Substitution in Square Planar Metal ComplexesThe animation shows a ligand substitution reaction of a square planar transition metal complex.For example: [PtCl4]2- + NH3 ---> [PtCl3(NH3)]- + Cl- The incoming ligand (coloured blue) approaches a vacant axial site of the square planar complex to form a square pyramidal intermediate (or transition state). Intramolecular rerrangement via a trigonal bipyramid generates a different square pyramidal structure with the incoming ligand now in the basal plane. (This motion is closely related to the Berry Pseudorotation mechanism) The reaction is completed by the leaving group departing from an axial site. Note that the stereochemistry of the complex is retained during the substitution process. This mechanism represents the "k2" pathway for direct associative substitution. A solvent assisted pathway can also operate, where the leaving group is first displaced by coordinating solvent, followed by rapid displacement of solvent by entering ligand. In certain circumstances a dissociative mechanism via a 3-coordinate intermediate can also occur (see lecture notes). |
|