Dr. Anthony Haynes
Department of Chemistry, University of Sheffield


|
Dr. Anthony HaynesDepartment of Chemistry, University of Sheffield |

|
|
|
|
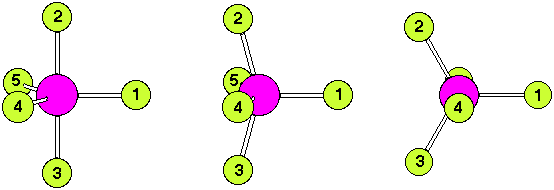
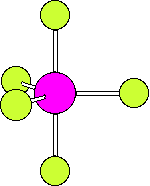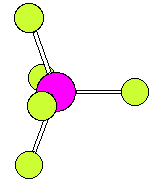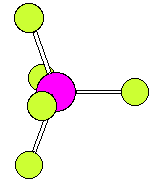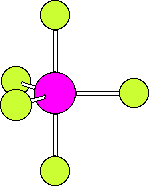
The numbering scheme is shown below. Ligands 2 and 3 move from axial to equatorial positions in the trigonal bipyramid whilst ligands 4 and 5 move from equatorial to axial positions. Ligand 1 does not move and acts as a pivot. At the midway point (transition state) ligands 2,3,4,5 are equivalent, forming the base of a square pyramid. The motion is equivalent to a 90o rotation about the M-L1 axis |
|